-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
SK Bioscience posts record earnings driven by COVID-19 vaccines
Earnings
SK Bioscience posts record earnings driven by COVID-19 vaccines
It aims to commercialize its own coronavirus vaccine in the first half, further boosting its sales revenue
By
Feb 08, 2022 (Gmt+09:00)
3
Min read
News+

SK Bioscience Co., a biopharmaceutical unit of South Korea’s SK Group, has posted record 2021 revenue and operating profit, boosted by contract manufacturing of COVID-19 vaccines for AstraZeneca plc and Novavax Inc.
The Korean vaccine maker’s sales increased more than fourfold to 929 billion won ($776 million) last year from 225.6 billion won in 2020. Operating profit soared to 474.2 billion won from 37.7 billion won, according to the company’s regulatory filing.
In the fourth quarter of last year alone, the company logged 451 billion won in revenue and an operating profit of 254 billion won, accounting for almost half its annual sales and profit.
SK Bioscience attributed its spectacular earnings performance to strong orders from its two global pharmaceutical clients for coronavirus vaccines under the contract manufacturing organization (CMO) scheme.
The Korean company produces British-Swedish pharmaceutical giant AstraZeneca’s COVID-19 vaccine under a drug product manufacturing deal, which involves putting the vaccine into vials or syringes, sealing and packaging for shipping.
Under a contract development and manufacturing organization (CDMO) deal with Novavax, SK is handling the entire production process, including making undiluted solutions or drug substances of the US company’s vaccine as well as vial filling.
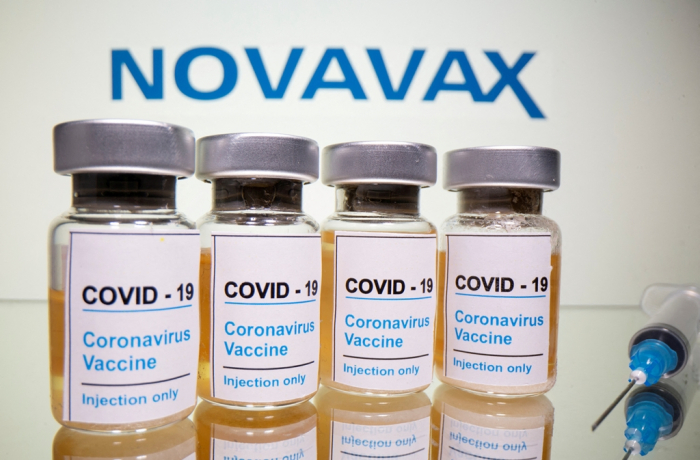
SK Bioscience said it expects to continue to post decent earnings this year, driven by contract orders from other companies and the sale of its own COVID-19 vaccine.
Last month, Korea’s Ministry of Food and Drug Safety approved the sale of Novavax’s COVID-19 vaccine, Nuvaxovid, in Korea -- a move that is expected to further boost SK Bioscience’s sales.
SK produces the synthetic antigen-based vaccine at its main plant in Andong, North Kyongsang Province.
“The protein-based vaccine has an advantage over mRNA-based vaccines, given that it can use existing distribution channels as it can be stored and distributed at much higher 2 to 8 degrees Celsius,” said an industry official.
In December of 2021, SK Bioscience said it acquired non-exclusive rights to sell Novavax’s COVID-19 vaccine, also known as NVX-CoV2373, to the governments of Thailand and Vietnam.
Under the extended agreement between SK and Novavax, SK’s rights to sell the Novavax vaccine will be valid until 2041 in Korea and 2026 in Thailand and Vietnam.
“We may clinch a supply deal with another country as early as this month,” said an SK Bioscience official.
TO SELL SK’S OWN COVID VACCINE IN H1
SK is currently developing its own recombinant coronavirus vaccine, which it aims to commercialize by the end of the first half.

The company said in August it received approval from Korea’s drug safety agency for the phase 3 clinical trials of its COVID-19 vaccine candidate GBP510.
It has jointly developed GBP510 with the Institute for Protein Design at the University of Washington under a project funded by the Bill & Melinda Gates Foundation and the Coalition for Epidemic Preparedness Innovations (CEPI).
SK Bioscience will continue to halt its flu vaccines this year to focus on manufacturing COVID-19 vaccines instead, according to industry sources.
The company said in March last year it is suspending the production of SKYCellflu, an influenza vaccine, to reallocate output capacity to produce coronavirus vaccines.
SK aims to start clinical tests for a combo vaccine that can protect people from both flu and coronavirus infections by year-end.
“We will spare no expense for the development of next-generation vaccines and vaccine platforms,” said SK Bioscience Chief Executive Ahn Jae-yong.
The company had 1.6 trillion won in cash reserves as of the end of 2021.
Write to Jae-young Han at jyhan@hankyung.com
In-Soo Nam edited this article.
More To Read
-
 COVID-19 vaccineSK Bioscience to sell Novavax COVID-19 vaccine in Thailand, Vietnam
COVID-19 vaccineSK Bioscience to sell Novavax COVID-19 vaccine in Thailand, VietnamDec 24, 2021 (Gmt+09:00)
-
 COVID-19 vaccineSK Bioscience to kick off phase 3 vaccine trials
COVID-19 vaccineSK Bioscience to kick off phase 3 vaccine trialsAug 10, 2021 (Gmt+09:00)
-
 Vaccine businessSK Bioscience halts flu vaccine output to focus on COVID vaccines
Vaccine businessSK Bioscience halts flu vaccine output to focus on COVID vaccinesMar 30, 2021 (Gmt+09:00)


