-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
Koh Young’s medical robot KYMERO expands foothold in Korean hospitals
Medical robots
Koh Young’s medical robot KYMERO expands foothold in Korean hospitals
KYMERO will enter Samsung Medical Center for surgical use, following its installation in Severance Hospital last year
By
Jul 07, 2021 (Gmt+09:00)
2
Min read
News+
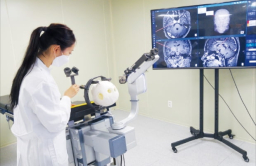
Koh Young Technology Inc., Korea’s leading maker of 3D optical inspection devices used in electronics manufacturing, has further strengthened its foothold in the medical robot market.
According to Koh Young on July 2, the company signed a deal with Samsung Medical Center, one of the leading hospitals in the country, to supply its medical robot KYMERO for surgical use.
KYMERO is the world’s first commercialization of a brain surgery robot that can be attached to the operating table. It uses a high-precision optical sensor to navigate the patient’s head and calculates the precise coordinates of the surgery accordingly.
The robot is used in stereotactic brain surgery, a sophisticated surgical procedure with an accuracy error of less than 1 millimeter, to treat hand tremors, Parkinson’s disease and epilepsy.
The deal with Samsung Medical Center marks the medical robot maker’s second partnership with major hospitals in the country, after successfully installing KYMERO at Yonsei University’s Severance Hospital in October last year.
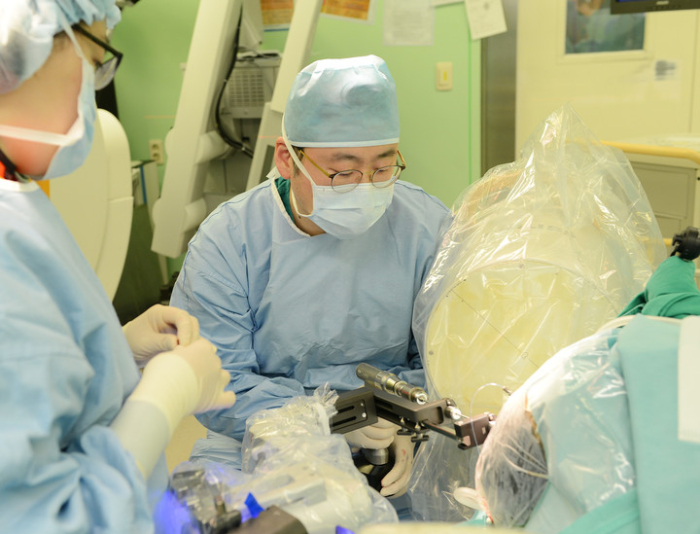
In April this year, KYMERO and a team of doctors at Severance Hospital led by Professor Jang Won-seok successfully completed Korea’s first robotic surgery to treat epilepsy.
Severance Hospital said that its doctors used KYMERO to insert electrodes into the brain of a 10-year-old patient with epilepsy, and removed the damaged part of the brain accordingly.
A DECADE-LONG EFFORT IN THE MEDICAL ROBOT SECTOR
Koh Young developed its first surgical robot KYMERO in 2011 as part of a Korean government-funded project assigned by the Ministry of Trade, Industry and Energy (MOTIE).
In 2016, the company received approval from the Ministry of Food and Drug Safety (MFDS) to produce and sell KYMERO in Korea.

After years of clinical trials, KYMERO first entered the hospital scene last year in Korea by signing an agreement with Severance Hospital.
Samsung Medical Center last year also conducted clinical trials of KYMERO and reported the final results in June 2020. The hospital, which started using robots in surgeries as early as 2008, is known as a pioneer in the country, leading the expansion of robotics in different medical fields.
“I carried out 25 brain surgeries with KYMERO as part of its clinical trials, in all of which KYMERO pointed to the exact lesion. KYMERO is expected to provide great assistance to the patients who cannot have drug treatments and who thus need surgical intervention,” said Samsung Medical Center’s neurosurgeon Dr. Lee Jung-il, who led the clinical tests of KYMERO.
Koh Young is planning to not only expand KYMERO’s application to other types of neurosurgeries but also enter overseas markets after receiving approvals from the relevant health authorities such as China’s National Medical Products Administration (NMPA) and the US Food and Drug Administration (FDA).
Koh Young added that the company will also file for CE marking in Europe so that KYMERO can be distributed within EU member countries.
Write to Dong-hyun Kim at 3code@hankyung.com
Daniel Cho edited this article.
More To Read
-
Jun 22, 2020 (Gmt+09:00)
-
Nov 24, 2020 (Gmt+09:00)