-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
Brazil gives emergency use nod for Celltrion’s COVID-19 treatment
Covid-19 treatment
Brazil gives emergency use nod for Celltrion’s COVID-19 treatment
Brazil marks the second country after Indonesia where the health authorities approved Regkirona for emergency use
By
Aug 13, 2021 (Gmt+09:00)
1
Min read
News+
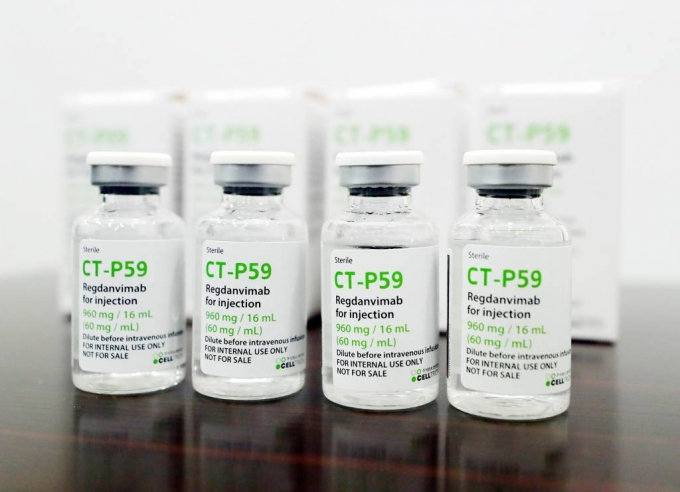
South Korean drugmaker Celltrion Inc. has added Brazil to the list of countries that have granted emergency use approval for its COVID-19 treatment Regkirona. Last month, the Indonesian government also gave permission for the restricted use of Regkirona in the country.
Celltrion said that the Brazilian health authorities on Aug. 11 approved Regkirona for high-risk adult patients with mild and moderate symptoms. The company added that the approval was based on the effective test results from its phase 1, 2 and 3 clinical trials as well as preclinical data on Delta and Gamma variants.
The number of confirmed cases in Brazil has been on the rise lately due to the fast spread of the delta variant. More than 35,000 patients were confirmed with COVID-19 in the country on Aug. 11 alone.
“Our company will accelerate the delivery of Regkirona to Brazil to help the patients to recover faster,” said a Celltrion official.
Celltrion is currently having a preliminary discussion with the Food and Drug Administration (FDA) in the US regarding the regulatory filing, while the European Medicines Agency (EMA) is conducting a review on Regkirona.
Prior to its official approval, the EMA in March granted Regkirona a recommendation to use by the EU member states. The recommendation allows European countries to use the treatment prior to the official outcome of the health regulator’s review.
Moreover, Pakistan’s state-owned company Wah Industries Ltd., purchased 100,000 vials of Regkirona in March to treat the country’s soldiers and the general public confirmed with COVID-19. Celltrion said the contracted amount of 100,000 vials is enough to treat 30,000 patients.
In South Korea, Celltrion has asked the Ministry of Food and Drug Safety (MFDS) to widen the scope of Regkirona’s use to include minors over the age of 12 as well as adults with mild and moderate symptoms. The Korean health regulator currently allows Regkirona to be used only on high-risk patients or those older than 60.
Write to Jae-young Han at jyhan@hankyung.com
Daniel Cho edited this article.
More To Read
-
 COVID-19 treatmentCelltrion to enter clinical trials of new COVID-19 treatment
COVID-19 treatmentCelltrion to enter clinical trials of new COVID-19 treatmentAug 05, 2021 (Gmt+09:00)
-
 Covid-19 treatmentIndonesia grants emergency use approval for Celltrion’s COVID-19 treatment
Covid-19 treatmentIndonesia grants emergency use approval for Celltrion’s COVID-19 treatmentJul 20, 2021 (Gmt+09:00)
-
 COVID-19 treatmentCelltrion’s COVID-19 treatment reduces incidence of severity by 70%
COVID-19 treatmentCelltrion’s COVID-19 treatment reduces incidence of severity by 70%Jul 13, 2021 (Gmt+09:00)


