-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
High-priced drugs add to pressure for Korea's healthcare reform
New rare disease treatments challenge S.Korea's expanded health insurance coverage
By
Sep 15, 2021 (Gmt+09:00)
A number of new rare disease treatments, approved in South Korea over the past year, are still unaffordable for the public because of their price tags of up to 2.5 billion won ($2.1 million) per dose.
The government's Health Insurance Review and Assessment Service on Sept. 1 put on hold its assessment of Novartis' Kymriah as a candidate for a blood cancer treatment to add to the national health insurance. If it is approved as a cancer or rare disease treatment, the government will shoulder 90-95% of the costs.
Novartis will complement its application for the new drug, designed to target cancer cells, to be covered by South Korea's national health insurance service.
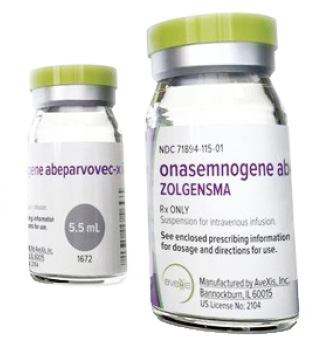
In regards to its new therapy of spinal muscular atrophy (SMA), or muscle weakness, the government drug review agency will soon discuss whether or not to add Zolgensma to the national health insurance, according to pharmaceutical industry sources on Tuesday.
The medication is regarded as an innovative treatment for SMA, the number one genetic cause of infant mortality, which can be cured with a single shot of Zolgensma priced at 2.5 billion won. But it is unlikely to be covered by the South Korean government anytime soon because it is the world's most expensive drug, according to industry sources.

Pfizer's Vyndamax heart medicine has been waiting one year to be approved for coverage under South Korea's national health insurance. It is used as a non-surgical treatment for those suffering from heart diseases who previously would need a heart or liver transplant.
These three new rare disease drugs are among the most expensive new treatments approved in South Korea over the past year.
Industry watchers said the healthcare industry has just entered the new era of high-priced prescription drugs, given that pharmaceutical companies are concentrating on the development of tailor-made treatments amid the growing demand for highly effective medications.
Most of the new treatments are expensive because they are produced in small quantities, unlike mass-produced high blood pressure treatments applied to a broader base of patients. In South Korea, they target only several hundred patients per year.
New Drugs Approved in South Korea
| Medication | Company | Disease | Price of a dose or a vial | Insurance coverage |
| Zolgensma | Novartis | Spinal muscular atrophy | 2.5 billion won | Not covered |
| Kymriah | Novartis | Blood cancer | 500 million won | Not covered |
| Vyndamax | Pfizer | Heart problems | 250 million won | Not covered |
| Keytruda | MSD | Stomach, lung and colon cancers | 5 million-6 million won | From second-line chemotherapy for non-small-cell lung cancer |
| Tecentriq | Roche | Lung, breast, liver and bladder cancers | 2.3 million won | For non-small-cell lung cancer, bladder cancer |
| Tagrisso | AstraZeneca | Lung cancer | 210,000 won | From second-line chemotherapy for non-small-cell lung cancer |
The high-priced treatments have put South Korea in a dilemma. Under the President Moon Jae-in administration, the government has expanded its healthcare coverage, sending the national health insurance balance into deficit for a third straight year.
Further widening its coverage to the rare disease medications means opening the floodgates to a spate of new expensive drugs, adding to pressure for national healthcare reform.
South Korea's health insurance reserves have shrunk to 17.4 trillion won as of end-2020, versus 20.8 trillion won in 2017. The government spent 20.9 trillion won to provide insurance coverage for drugs in 2019, up 54.2% from the 13.5 trillion won spent in 2010.
Write to Sang-hun Oh and Sun-A Lee at ohyeah@hankyung.com
Yeonhee Kim edited this article.
-
 CryptocurrenciesRedotPay lands in Seoul with crypto cards
CryptocurrenciesRedotPay lands in Seoul with crypto cardsMay 09, 2025 (Gmt+09:00)
-
May 09, 2025 (Gmt+09:00)
-
 Corporate strategyHankook & Company to set up CVC with $11 mn in funding
Corporate strategyHankook & Company to set up CVC with $11 mn in fundingMay 09, 2025 (Gmt+09:00)
-
 Electric vehiclesBYD’s Atto 3 overtakes Tesla’s Model Y as best-selling EV in South Korea
Electric vehiclesBYD’s Atto 3 overtakes Tesla’s Model Y as best-selling EV in South KoreaMay 09, 2025 (Gmt+09:00)
-
May 08, 2025 (Gmt+09:00)




