-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
HealthQuest-backed Lunit applies for 2022 Kosdaq IPO
Lunit's OTC market cap tops 1 trillion won, double the valuation pinned by VCs this month
By
Nov 30, 2021 (Gmt+09:00)
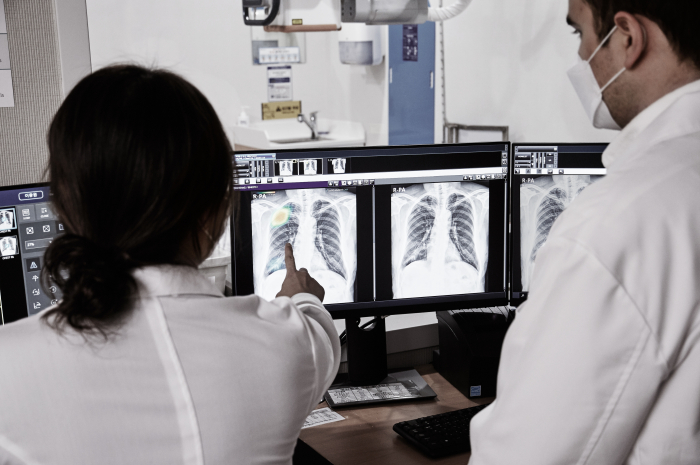
Lunit Inc., a South Korean developer of AI-based diagnostic tools for cancer screening, has surpassed 1 trillion won ($841 million) in value in over-the-counter markets, as it is gearing up for an initial public offering next year.
Lunit applied for a preliminary review for its public offering with the Korea Exchange on Nov. 26, according to the stock exchange on Monday. It plans to float 1.49 million shares on the junior Kosdaq market in 2022, or 12% of its shares outstanding. NH Investment & Securities Co. is the lead manager.
The news of its IPO application immediately drove its shares traded in OTC markets more than 30% higher to exceed 100,000 won in less than a month.
Based on the price, its corporate value is estimated at 1.1 trillion won, more than double the 500 billion won pinned by foreign venture capitalists, including the US-based HealthQuest Capital, when it raised $61 million in a pre-IPO placement earlier this month. Lunit became HealthQuest's first investment in Asia.
VALUATION
Investment bankers said that at an IPO, Lunit's enterprise value would be proposed at a minimum of 700 billion won by the VCs to facilitate their exit from the Korean startup.
Last July, Lunit received a strategic investment of $26 million from Guardant Health, a cancer diagnostics company, in Series D funding. Lunit has secured more than $135 million in total to date.
Lunit was founded in 2014 by six members of a hip-hop club at the Korea Advanced Institute of Science & Technology (KAIST). It develops artificial intelligence-based radiology software for chest X-ray and mammography in partnership with medical device companies, including GE Healthcare, Philips, and FujiFilm.
The company develops deep learning models for diagnostic medical imaging to increase the accuracy of image interpretation. It also boasts cutting-edge technology for artificial neural network algorithms, inspired by the structure of the brain.

In a recent assessment of its technology by evaluation agencies, a process required for a startup to qualify for a domestic IPO, Lunit was given the highest rating of double-A. It became the first healthcare company to receive the highest scores in the technology assessment among unlisted Korean startups.
However, the company saw its operating loss nearly doubling to 21 billion won in 2020 from a year before, with a net loss of 83.7 billion won on sales of 1.4 billion won last year. Its liabilities amounted to 155.8 billion won as of end-2020.
This year the company expects a sevenfold-increase in sales to 10 billion won.
OTHER KOREAN HEALTHCARE VENTURES
On Kosdaq, JLK Inc., an AI-based medical imaging products developer, has been traded since 2019. Its share price has slid to 5,000 won with a market capitalization of 90 billion won, compared with its listing price of 9,000 won.
Vuno Inc., another AI-driven diagnostic software developer, is now below its IPO price with a market capitalization of 200 billion won.
"Medical device companies have not been favorites in local stock markets. Shares in listed AI-based medical device companies have been underperforming, raising questions about their industry," said one of the investment banking sources.
"For Lunit to be recognized as a unicorn, it must generate sales from overseas markets, or prove its market competitiveness by entering partnerships with a global hospital or pharmaceutical company," he noted.
Last week Lunit said its radiology solution for chest X-ray and mammography, dubbed Lunit INSIGHT, is commercial in more than 30 countries and will also be offered in the US after it recently won approval from the US Food and Drug Administration.
Write to Ye-jin Jun at ace@hankyung.com
Yeonhee Kim edited this article
-
Nov 24, 2021 (Gmt+09:00)