-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
Kolon resumes Invossa phase 3 clinical trials in US
Pharmeceuticals
Kolon resumes Invossa phase 3 clinical trials in US
After an ingredient mislabeling scandal, Kolon has won approval to continue phase 2 and 3 studies on the drug's different targets
By
Dec 28, 2021 (Gmt+09:00)
1
Min read
News+
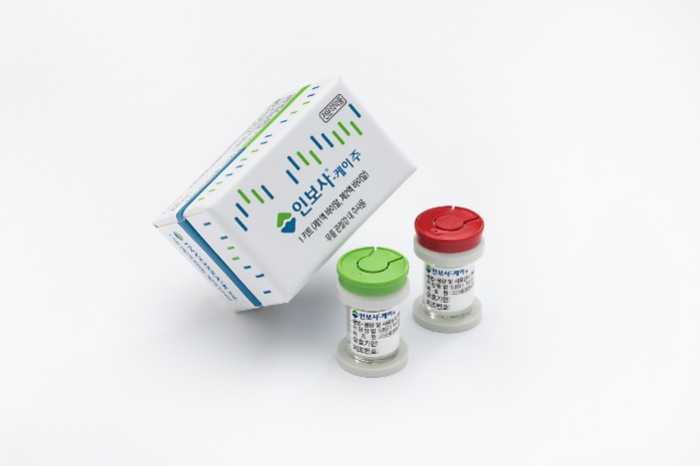
The Ministry of Food and Drug Safety (MFDS) of Korea approved the drug in July 2017 as a cell and gene therapy for patients with degenerative joint disease. However, the health authorities canceled the drug licensing in March 2019, following a scandal that Kolon had manufactured the drug with a main ingredient different from that labeled at the time of approval.
According to the MFDS, the drug was labeled as using cartilage-derived cells, but it was actually using kidney-derived cells. In May 2019, the US Food and Drug Administration suspended the drug’s phase 3 clinical trials.
Kolon Life Science appealed to suspend the execution of MFDS’ order to stop manufacturing Invossa, however, the Seoul Central District Court dismissed the appeal in February of this year.
In April 2020, FDA allowed Kolon TissueGene to resume clinical trials of Invossa after reviewing supplementary data from the Korean firm, including an explanation of how the drug composition has changed and additional data on the cell ingredients. Earlier this month, FDA approved Invossa’s phase 2 clinical trial for hip joint osteoarthritis, for the drug’s target expansion.
“We expect a positive result from the phase 3 clinical trials as the scientific data from phase 1 and 2 clinical trials have high reliability and validity. By completing the clinical study successfully, we will make Invossa a game-changer in the global osteoarthritis therapy market,” said Kolon TissueGene Chief Executive Sung Han.
Write to Ju-Hyun Lee at deep@hankyung.com
Jihyun Kim edited this article.
More To Read
-
 COVID-19 vaccineSK Bioscience to sell Novavax COVID-19 vaccine in Thailand, Vietnam
COVID-19 vaccineSK Bioscience to sell Novavax COVID-19 vaccine in Thailand, VietnamDec 24, 2021 (Gmt+09:00)
-
 PharamaceuticalsSK Biopharm releases epilepsy treatment in UK
PharamaceuticalsSK Biopharm releases epilepsy treatment in UKDec 21, 2021 (Gmt+09:00)
-
 Shareholder activismActivist fund again urges SK Chem to sell SK Bioscience stake
Shareholder activismActivist fund again urges SK Chem to sell SK Bioscience stakeDec 17, 2021 (Gmt+09:00)
-
 COVID-19 vaccineKorea approves export of Samsung Biologics-made Moderna vaccine
COVID-19 vaccineKorea approves export of Samsung Biologics-made Moderna vaccineDec 14, 2021 (Gmt+09:00)
-
 PharmaceuticalsSamsung Biologics wins another Eli Lilly COVID treatment order
PharmaceuticalsSamsung Biologics wins another Eli Lilly COVID treatment orderNov 19, 2021 (Gmt+09:00)
-
Oct 21, 2020 (Gmt+09:00)



