-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
Korean bio firms look to pills, patches for people who fear needles
Bio & Pharma
Korean bio firms look to pills, patches for people who fear needles
Biopharmas are working on alternative drug formulations that have the same efficacy and safety as injections
By
Aug 24, 2022 (Gmt+09:00)
2
Min read
News+

Getting shots of a drug or vaccine over a long period is not easy for many people.
That's probably why global biopharmaceutical companies and biotech firms are eager to develop easy methods of administering drugs that have the same efficacy and safety as injection therapeutics.
South Korean biopharmaceutical firms are following suit with their alternative drug formulations for those who fear needles.
Alteogen Inc., a local bio company, has developed a platform that mixes an anticancer drug with hyaluronidase, an enzyme that breaks down hyaluronic acid in the body, beneath the skin so that the drug can easily enter the blood vessels.
The company has already exported its biotechnology to three global biopharmaceutical companies, a deal worth a combined 6 trillion won ($4.5 billion).
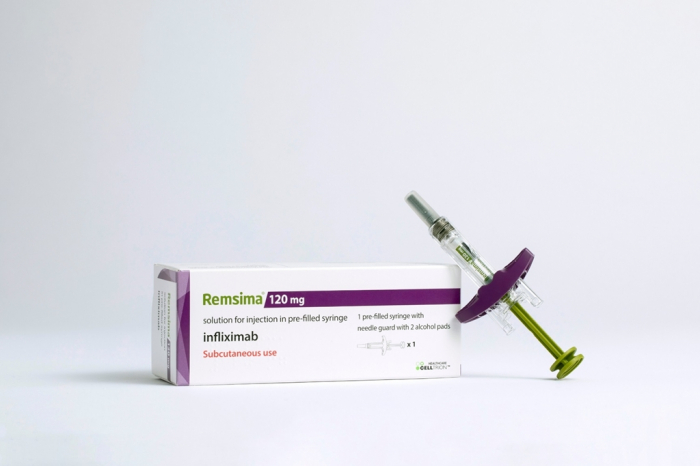
Celltrion Inc., which unveiled the world’s first subcutaneous (SC)-injection type of Remsima, a biosimilar version of Janssen’s Remicade, aims to bring the medicine to the US market next year.
In Europe, Celltrion’s Remsima SC, used for the treatment of rheumatoid arthritis, accounted for 9.1% of the market in the first quarter.
Sam Chun Dang Pharm. Co. is developing an insulin pill, using its own oral dosage technology called S-PASS. The company plans to carry out global clinical tests by the end of this year.
Raphas Co. is developing dissolving microneedle patches for therapeutics and vaccine administration.
The company’s microneedle patch uses the mechanism of percutaneous delivery of drugs by attaching a patch embedded with microneedles to the skin.
GLOBAL TREND
Earlier this month, global pharmaceutical company Roche proved that the subcutaneous injection formulation of Tecentriq, an immunotherapy cancer and lung cancer treatment, is not inferior to the existing intravenous injection method, during its phase 3 clinical trials.
Roche used US biotech firm Halozyme Therapeutics’ technology that mixes an anticancer drug with hyaluronidase.
Merck Sharp & Dohme Corp. (MSD) and Pfizer Inc. are also testing such methods through their phase 3 clinical trials with the goal of completion by 2023 and 2024, respectively.
Merck's Keytruda is one of the world's best-selling anticancer drugs, but it needs to be administered intravenously (IV) for 30 minutes every three weeks.
“People prefer pills and patches over shots as long as such methods have the same effect. That’s why bio companies are developing alternative drug formulations one after another,” said an industry official.
Write to Jae-young Han at jyhan@hankyung.com
In-Soo Nam edited this article.
More To Read
-
Aug 19, 2022 (Gmt+09:00)
-
May 11, 2022 (Gmt+09:00)
-
Nov 16, 2021 (Gmt+09:00)
-
Sep 23, 2021 (Gmt+09:00)






