-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
HK inno.N gets approval to sell K-CAB in Singapore
Bio & Pharma
HK inno.N gets approval to sell K-CAB in Singapore
This is the fifth overseas permit for the ulcer treatment after Mongolia, China, the Philippines, and Indonesia
By
Jan 12, 2023 (Gmt+09:00)
1
Min read
News+
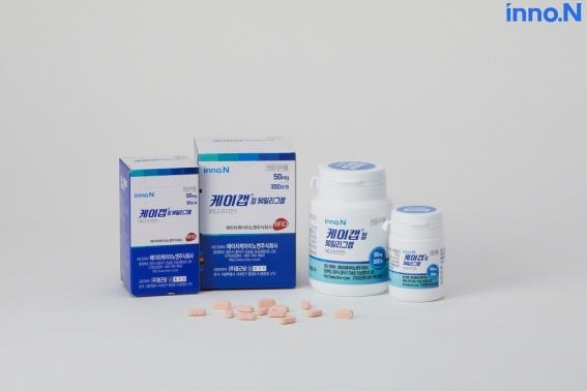
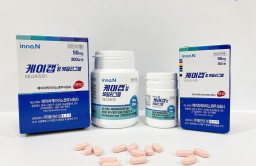
South Korean biohealth company HK inno.N said on Wednesday that it has received an item approval for K-CAB, a new drug for gastroesophageal reflux disease (GERD), from Singaporian authorities.
K-CAB is the 30th domestic new drug released by HK inno.N in 2019 and is a new potassium competitive acid blocker (P-CAB). It became a blockbuster drug that recorded more than 100 billion won ($80 million) in outpatient prescriptions per year in Korea.
It has been licensed in Singapore to treat GERD and has obtained indications for erosive GERD, non-erosive GERD, gastric ulcer, and Helicobacter pylori eradication in combination with antibiotics.
This is the fifth overseas permit after Mongolia, China, the Philippines, and Indonesia.
In 2020, HK inno.N signed an exclusive distribution contract with its Singapore partner UITC for eight years after its local launch of K-CAB.
"The latest approval will accelerate our pace to enter the Southeast Asian market and expand overseas sales. We will develop K-CAB into a global blockbuster drug,” said Kwak Dalwon, CEO of HK inno.N.
Write to Ji-Hyun Lee at bluesky@hankyung.com
More To Read
-
Apr 13, 2022 (Gmt+09:00)
-
 PharmaceuticalsHK inno.N’s K-CAB a blockbuster drug with $843 million exports
PharmaceuticalsHK inno.N’s K-CAB a blockbuster drug with $843 million exportsDec 24, 2021 (Gmt+09:00)
-
Jul 30, 2021 (Gmt+09:00)