-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
SK Biopharmaceuticals gets marketing approval for epilepsy drug in Israel
Bio & Pharma
SK Biopharmaceuticals gets marketing approval for epilepsy drug in Israel
The S.Korean pharmaceutical company plans to expand Cenobamate into West Asian markets
By
May 03, 2023 (Gmt+09:00)
1
Min read
News+
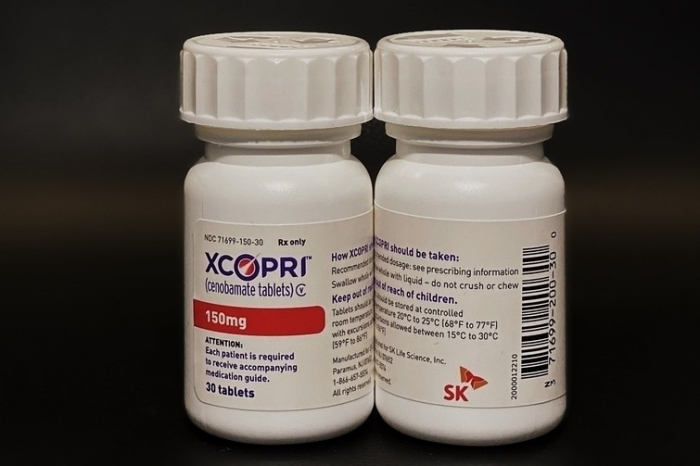
South Korea’s SK Biopharmaceuticals said on Wednesday that the Israeli Ministry of Health has approved the company's new drug application (NDA) for its epilepsy medication Cenobamate (local name Ecopri) on April 30.
The application was submitted by Dexcel Pharma, the Israeli partner of SK Biopharmaceuticals. The approved drug includes a total of six dosages: 12.5 mg, 25 mg, 50 mg, 100 mg, 150 mg and 200 mg.
Cenobamate, an independently developed epilepsy drug by SK Biopharmaceuticals, was recognized for its efficacy in treating partial-onset seizures in adults and received marketing approval from the US Food and Drug Administration (FDA) in November 2019.
Following the recent approval, SK Biopharmaceuticals stated plans to accelerate its market strategy in Western Asia, including Israel.
Write to Yoo-Rim Kim at youfrest@hankyung.com
More To Read
-
Feb 16, 2023 (Gmt+09:00)
-
Dec 09, 2022 (Gmt+09:00)
-
Nov 11, 2022 (Gmt+09:00)
-
Jul 15, 2022 (Gmt+09:00)
-
 PharamaceuticalsSK Biopharm releases epilepsy treatment in UK
PharamaceuticalsSK Biopharm releases epilepsy treatment in UKDec 21, 2021 (Gmt+09:00)