-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
Theraject Asia applies for microneedle patent in US
Bio & Pharma
Theraject Asia applies for microneedle patent in US
The S.Korean company’s static pressure syringe technology utilizes its expertise in drug-injecting microneedles
By
Oct 27, 2023 (Gmt+09:00)
1
Min read
News+
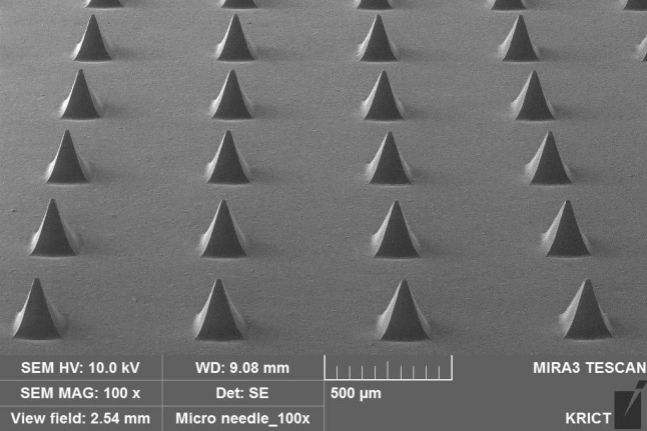
South Korean microneedle company Theraject Asia on Thursday said it recently applied for a US patent for a medical microneedle, specifically a constant pressure model that controls active drug penetration and resolves effect reproducibility.
As a system for delivering transdermal drugs using a microneedle hundreds of micrometers long, a microneedle can administer a variety of medicines depending on its size, strength and material. Though microneedles are growing in prominence as a cutting-edge technology, know-how in standardized transdermal injection is needed for drug reproducibility when applied to the skin.
Theraject Asia has secured technology for static pressure syringes that combines know-how in drug-injecting microneedles, enabling easy application to the skin under standardized physical conditions.
The company on Wednesday signed a joint research agreement with JW Pharmaceutical to use this technology for development of a hair loss treatment using microneedles.
“In 2019, we received from Theraject in the US an exclusive license for the original patent for a dissolvable pharmaceutical microneedle,” Theraject Asia CEO Kim Kyeong-dong said. “Since 2020, our focus has been finding methods to overcome obstacles to commercial application of a medical microneedle patch.”
Write to Ji-Hyun Lee at bluesky@hankyung.com
More To Read
-
 Bio & PharmaRaphas microneedle passes US FDA's cGMP inspection
Bio & PharmaRaphas microneedle passes US FDA's cGMP inspectionJul 26, 2023 (Gmt+09:00)
-
Apr 19, 2023 (Gmt+09:00)
-
Mar 27, 2023 (Gmt+09:00)




