-
KOSPI 2577.27 -2.21 -0.09%
-
KOSDAQ 722.52 -7.07 -0.97%
-
KOSPI200 341.49 +0.02 +0.01%
-
USD/KRW 1396 -2.00 0.14%
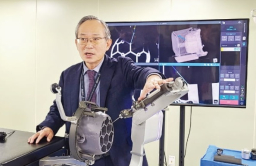
Koh Young gets OK from FDA for brain surgery robot
Robotics
Koh Young gets OK from FDA for brain surgery robot
The company aims to expand overseas medical surgery robot business, valued at $12.7 bn
By
Jan 21, 2025 (Gmt+09:00)
1
Min read
News+
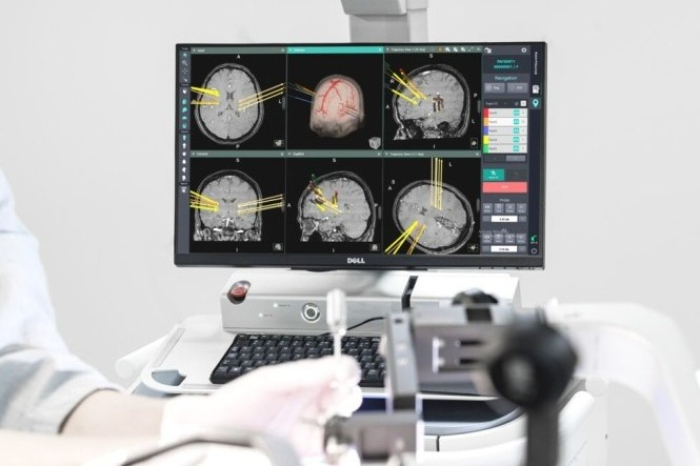
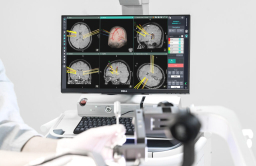
Geniant Cranial is a stereotactic robotic guidance system that uses medical imaging to support complex procedures, including surgeries for epilepsy and Parkinson’s disease, brain tumor biopsies, and intracerebral hemorrhage operations.
Koh Young Technology is the number one company dedicated to developing 3D solder paste inspection (SPI) equipment.
The company developed its first surgical robot, Kymero, in 2011 as part of a Korean government-funded project assigned by the Ministry of Trade, Industry, and Energy. In 2016, the company also received approval from the Ministry of Food and Drug Safety to produce and sell Kymero in South Korea.
After conducting clinical tests in South Korea, Koh Young Technology signed a contract with Yonsei University’s Severance Hospital in June 2020 to supply Kymero for surgical uses.
According to the Korea Institute of Science and Technology Information, the global robot market is expected to more than double to $12.7 billion with North America accounting for 62% by 2025 from $5.9 billion in 2020.
Write to Jong-Hwan Won at won0403@hankyung.com
More To Read
-
May 09, 2024 (Gmt+09:00)
-
Mar 15, 2023 (Gmt+09:00)